CBD infusion into edible products
CBD, THC, general alkaloids, and general cannabinoids market have increased greatly in the last decade, especially in most US states and some EU regions. During the last 5 years especially, CBD, various alkaloids and cannabinoids have flooded the market, mostly in novel formulations and edibles’ variations, as for example:
- Cakes
- Cookies
- General snacks
- Soft gel encapsulated products
- Jellies
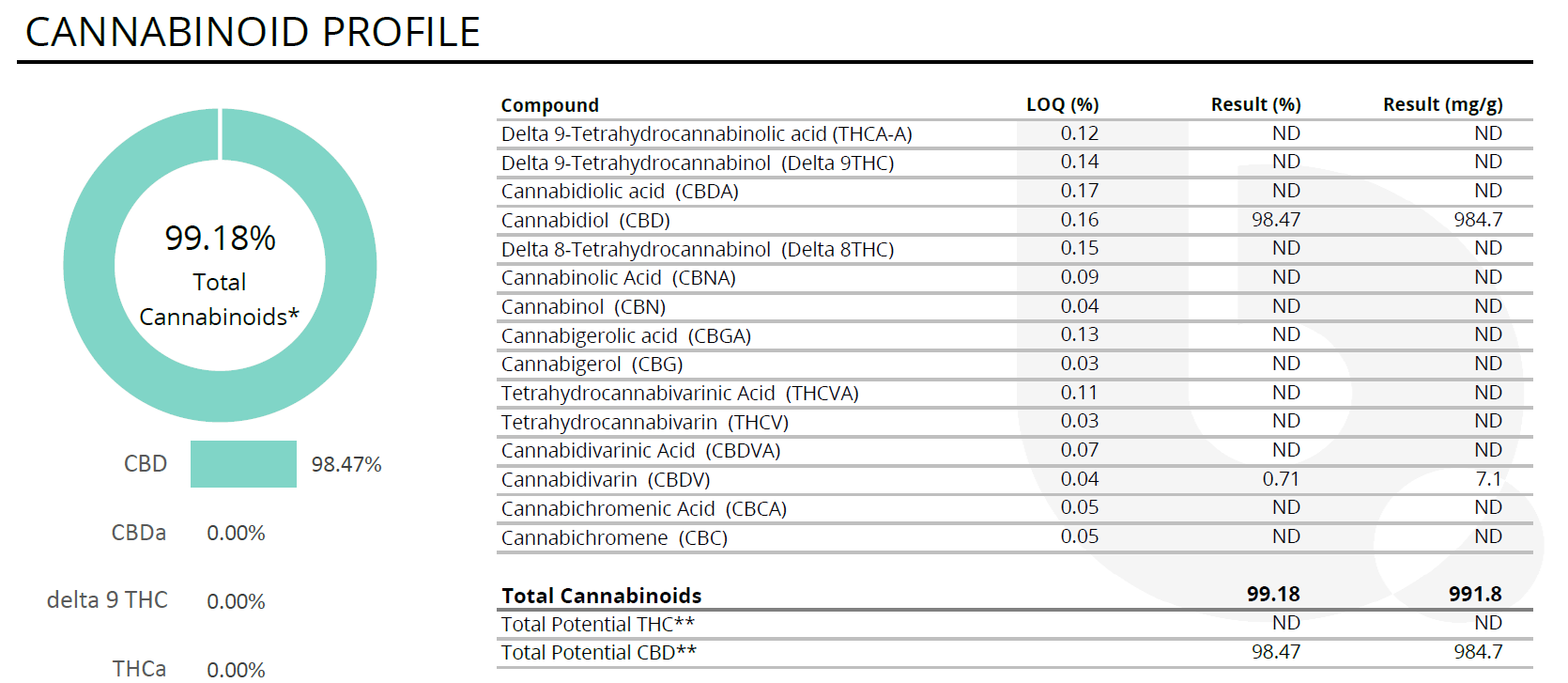
- Gummy bears
- Soft drinks
The industry took advantage of partial and local legalization of CBD and its ‘family’ components to create new versions of popular edibles (mainly) with partial contents of 5 – 70 mg per serving. While there are still many considerations about legality and future health effects, the market keeps growing world- wide. Infusion of CBD (and the rest of cannabinoids and alkaloids) takes place into edibles via different chemistries:
n Via carrying with organic, non-polar solvents and lipids
n Via encapsulation in specific macromolecules that possess both hydrophilic and oleophilic character
n Via water- based formulations, using specific carrier molecules as in the previous cases, for example Cyclodextrins (α- , β- γ-, hydroxy-, etc.) in combination with very efficient dispersions techniques such as:
- Supersonication
- High Pressure Homogenization (HPH)
- Microfluidic Homogenization (MH)
All of the above work optimally with the help of suitable dispersion agents and surfactants, and most commonly commercial solutions exist (Tween 40, Tween 80, etc.)
The infusion case (almost every time) takes place during manufacture, by simple addition of the actives (CBD, alkaloids, etc.) into the production mixture in the form of a pre- manufactured and stabilized emulsion. Lipophilic carriers can be used when required, and the process is quite known. Several manufacturers of such stable emulsions exist in the market today.
To sum the easy, during manufacture infusion of CBD onto an edible, the following steps are described:
- If edible contains mainly lipophilic groups and content, the CBD amount (usually less than 70 mg per serving) is mixed directly with the simultaneous use of a food grade surfactant and in many cases it is carried into the formulation mix on a food grade alcohol, fatty or lipid mix. This simple approach is used for cakes, cookies, some sauces, etc. The advantages include:
- Very low complexity of the process
- No need for emulsification (thus low cost process)
- Very fast process (thus low cost, high throughput)
Some disadvantages include:
- No control over the actual size distribution, and thus bioavailability and bio absorption
- Can only work with highly lipid and fat contents
- In cases with significant content of carbohydrates and proteins, as well as water content, the situation is completely different. Top manufacturers and CBD technological contractor definitely choose various emulsion – based solutions. Those solutions are much preferrable for ANY formulation since:
- They exist in much different compositions to allow for different CBD loading
- They exist with or without taste, for use on demand
- They can MODIFY and CONTROL CBD particle sizes, and thus regulate release, bio- absorption and bio- availability
- They allow for excellent control of CBD amount without fluctuations that can lead to legislative concerns and authorities’ investigations.
However, our client requested for a post- manufacture infusion of CBD onto edibles; post manufacture addition presents some marketing advantages such as the no- degradation of the active as it could happen during a simultaneous manufacturing and mixing. However, the actual process is more demanding one, and to our knowledge no other product in the market was manufactured in the post- manufacture infusion.
We examined and investigated all the potential methodologies for a stable, controlled infusion of CBD, in doses of 5- 60 mg per serving onto cookies and gummy bears. Out of the six total candidates, the most suitable was chosen, and the device selection followed. Currently, the infusion of edibles is in R&D stage, with very promising results. The CBD infused edibles are expected to enter the market within the first half of 2022.
