Explosive potential calculation of Tetrafluoro Hydrazine
Our clients asked us to estimate the explosive potential of a known unstable chemical, Tetrafluoro hydrazine. Tetrafluoro hydrazine is a gas oxidizer, that participates in explosive combinations but it is not itself an ‘explosive’. This fact made this search more complicated and the various approaches to calculate the explosive nature of it are presented in this short report. A tool was created where the user can modify the parameters of the system and calculate the explosivity attributes.
There are different groups of explosive materials based on their ‘sensitivity’ and total energy released. The most sensitive compounds are used as initiators, whereas the most powerful ones are used as the main components of explosive combinations and formulations. The most widely accepted classification of explosive compounds includes the following groups:
- Compounds containing nitrogen- oxygen bonds (nitrates, nitric esters, nitroaliphatics, and nitroaromatics)
- Peroxides and ozonides
- Chloroderivatives such as chloramines, oxy chloro acids, and their salts
- Self-linked nitrogen compounds including hydrazines, nitramines, and azo-, diazo- and azido- compounds
- Fluoro derivatives including difluoro amines and fluoro nitro compounds
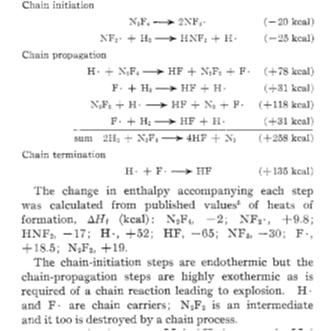
Although Tetrafluoro hydrazine typically could fall into this classification, it is hardly recognized as an explosive. An explosion requires the reaction of a ‘fuel’ with an oxidizer to produce great amounts of energy at a very short time. This release of energy and mass (volumes produced are higher than those reacted) creates a blast wave with catastrophic effects. Tetra fluoro hydrazine is not the fuel in any similar scheme; on the contrary, it is known to react violently with other fuels such as hydrogen, methane, and other hydrocarbons. Research on the instability of tetra fluoro hydrazine has revealed that:
n It can explode at a range of pressures if oxygen or moisture is present, even at ppm concentrations. Note however that according to properties’ databases (for example PubChem) and manufacturers (for example CAMEO chemicals), ‘Tetrafluoro hydrazine does not react rapidly with water or oxygen’
n Many inhibitors exist for these explosive schemes, and the most common ones are long chain hydrocarbons
n The reaction scheme is always a free radical scheme, which confirms the explosive features of the reaction scheme
n Tetrafluoro hydrazine can de- compose thermally or under shock. This would confirm the ‘instability’ experienced and reported in many cases
n It is highly explosive when in contact with a range of fuels, including alcohols, amines, ammonia, beryllium alkyls, boranes, dicyanogen, hydrazines, hydrocarbons, hydrogen, nitroalkanes, powdered metals, silanes, and thiols
n Can explode at high temperatures
n Can explode at high pressures under shock
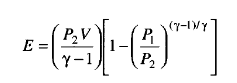
In order to evaluate the blast characteristics of any explosive, three factors have to be considered:
1- The actual explosive potential of a compound based on the energy released and the rate at which this energy is released when the combustion with an oxidizer takes place
2- The actual stored energy due to pressure applied in a store medium such as a pressurized gas cylinder and the rate at which this stored energy will be released at the event of a crack
3- The self- or assisted decomposition reactions that can lead to an energy and volumes generation, and the rates of the energy and volumes generation
The second case, which is of interest, pertains to the actual stored energy of a compressed gas in a vessel. This stored energy can be released at the event of a vessel crack OR in the event of an induced decomposition of the contained fluids that breaches the pressure safety limit of the container vessel. Typically, four methods are used to calculate the explosion attributes for the pressurized fluids:
a- Brode
b- Isentropic
c- Isothermal
d- Availability
Brode’s method is the simplest, most straight forward method to calculate the explosion energy and is described as:
The isentropic calculation/ method assumes an isentropic expansion to the final state:
The isothermal method/ calculation assumes an isothermal expansion to the final state:
The ‘availability’ method accounts for the losses in energy as the explosive systems comes into equilibrium with the surroundings via thermal transfer and thus, the available explosion energy is lower, as seen by the introduced correction term:
We proceeded with accurate calculations in MATLAB and in excel to provide an automated system for the explosive potential of Tetrafluoro Hydrazine in terms of:
- Blast wave determination
- Blast wave pressure generation
- Pressure profile
- Correlation between the actual kinetics and the power released in each scheme
- Safe distances from explosion points
|
