Computational Chemistry in Education sector
Computational Chemistry [Theoretical Chemistry] can be a powerful tool in educational sector, for both beginners and experts in the fields of Chemistry, Chemical Engineering, Physics, Material Science and others. Some of the first barriers in the learning process of chemistry are how to perceive the chemical structures, the different approaches to model these chemical structures, and the mechanisms that chemical compounds use to react with each other. Computational Chemistry tools make this learning process easy, well understood and fun.
When students are first taught about chemical structures, they have to understand and visualize a system that makes sense to them; listening about Kekule structures, electronic configurations and other approaches help, but does not provide a means for in-depth and quick understanding of chemical structures. On the other hand, using a computational chemistry tool with a graphical user interface helps students to understand the physical and chemical structure of chemical elements and compounds, rotate them in three dimensional space, understand bond lengths and angles, and familiarize themselves with a new reality: chemistry.
Working with Benzene following a typical educational approach requires that students understand planar structures, hybrid bond lengths, equal distribution of electronic charges, and hexagonal geometry of Benzene. Using however a computational chemistry tools with a graphical user interface [GUI] like HyperChem, HyperCube or Spartan, Wavefunction allows for:
- Step by step creation of Benzene
- Model building of Benzene
- Optimization of Benzene [more details on that in another part]
- Investigation of physical, chemical and geometrical attributes of Benzene
- Use of Benzene into more advanced simulations
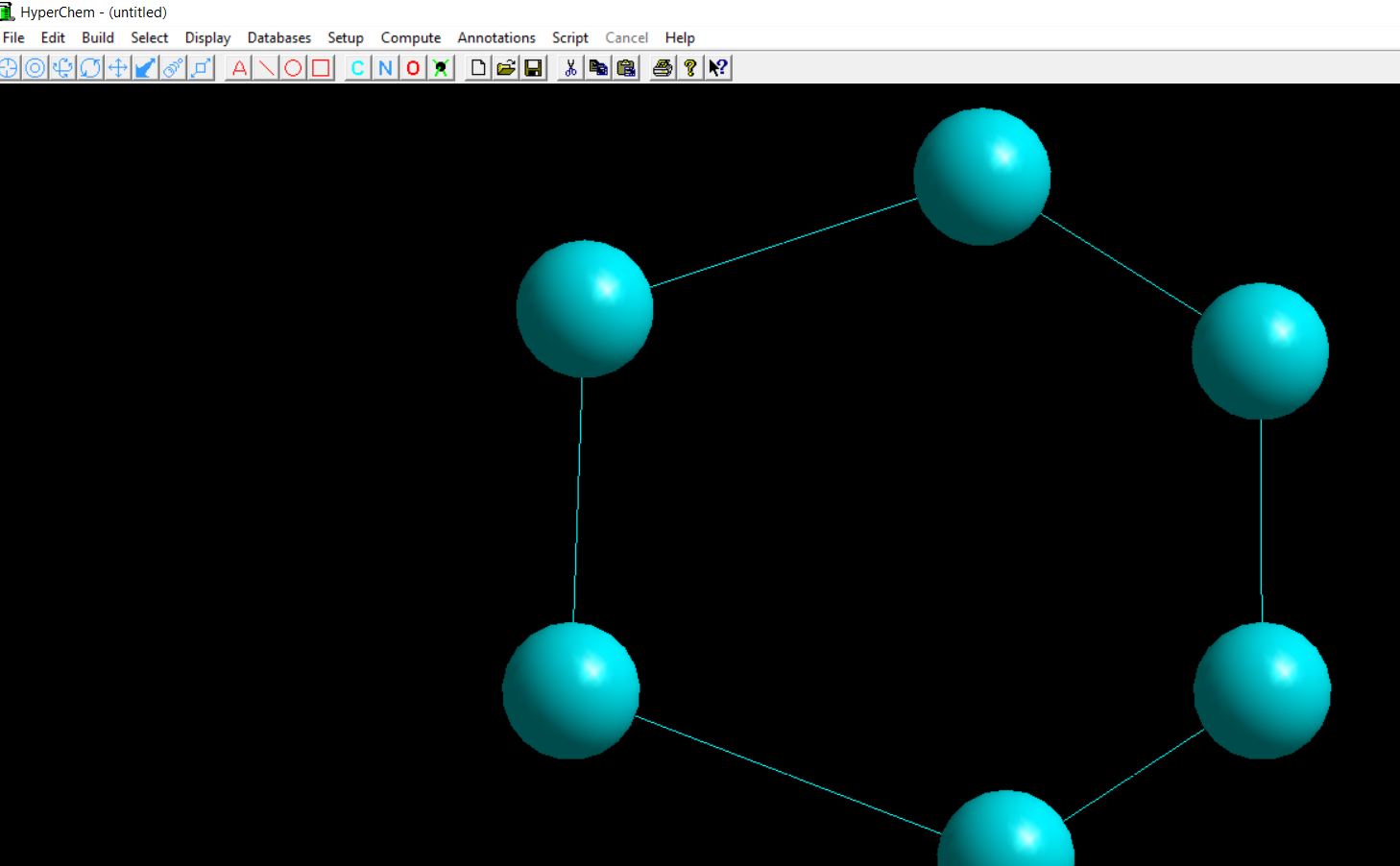
Building Benzene starts with connecting six carbon atoms as seen in first figure; then three double bonds are added; next step is to build the Benzene the model; with the pressing of just two buttons, the electrostatic potential in 2D [or 3D] is presented on the screen!
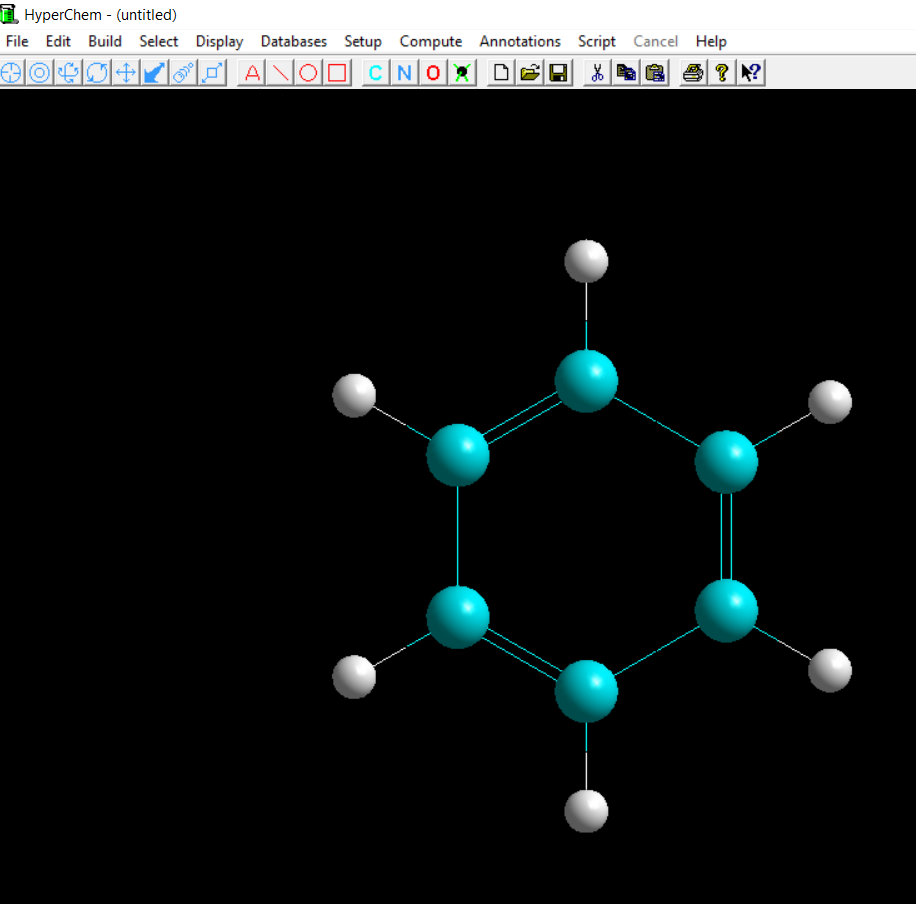
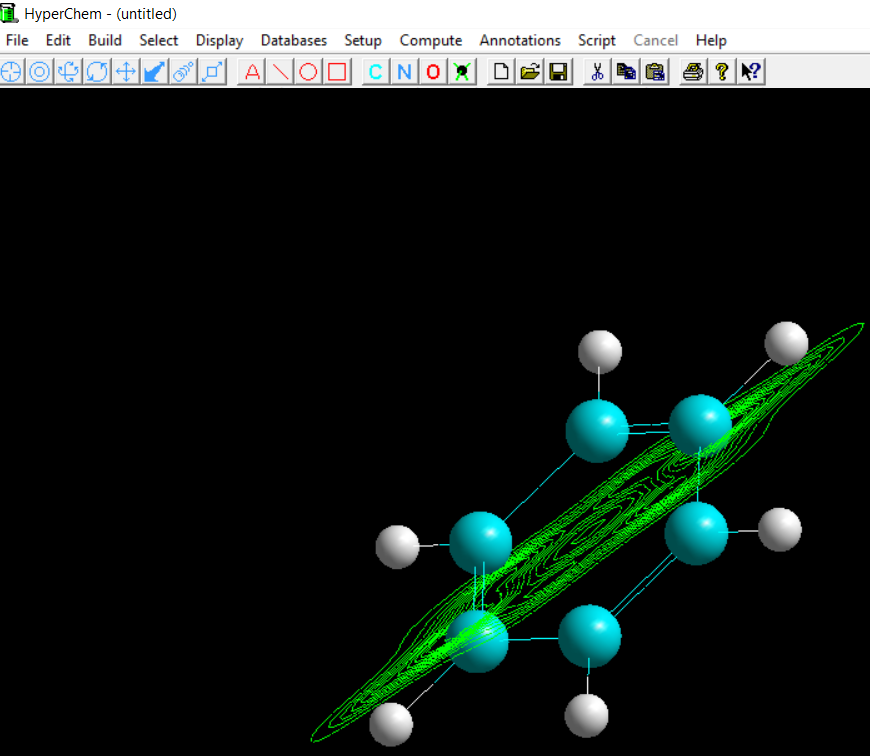
The whole process takes less than 30 seconds and reveals every aspect of benzene structure that a student should know:
- By rotating the molecule one can see and comprehend the planarity of benzene molecule
- By choosing two atoms one can see the size of their distance or bond if they are connected
- By choosing three different atoms one can see the angle they form
- By choosing one atom one can see the element, the mass, the charge and coordinated of that atom
- By investigating the electrostatic potential and charge distributions in 2D or 3D one can understand chemical reactivities and reaction mechanisms
- Tens of other properties [kinetics, thermodynamics, physical, chemical, electronic, etc] are obtained instantly
Students can experiment with the build models and acquire deeper insight of molecular and atomic properties. Modification of structures/ models provides the absolute tool to understanding functional groups, reactivity, and properties of compounds. The potential of theoretical chemistry tools in education is vast; more of that will be presented in upcoming posts!
